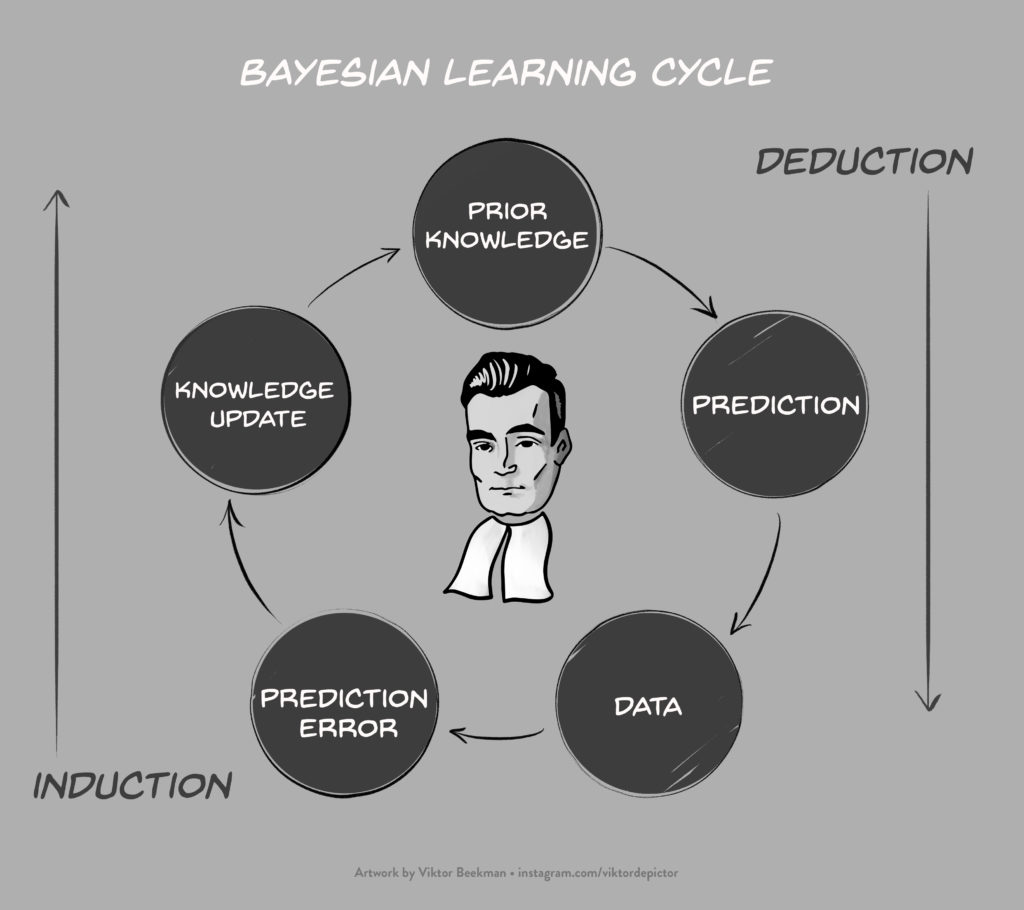
“Bayesian Inference Without Tears” at CIRM

Today I am presenting a lecture for the “Masterclass in Bayesian Statistics” that takes place from October 22 to October 26th 2018, at CIRM (Centre International de Rencontres Mathématiques) in Marseille, France. The slides of my talk,“Bayesian Inference Without Tears” are here. Unfortunately the slides cannot convey the JASP demo work, but the presentations are taped so I hope to…
read more